Abstract
Background: Children with Down syndrome (DS, trisomy 21, T21) are at increased risk for transient myeloproliferative disease (TMD) and acute megakaryoblastic leukemia (AMKL). Somatic mutations of GATA1, a critical hematopoietic transcription factor, lead to exclusive expression of an N-terminal truncated protein GATA1short (GATA1s). In DS, GATA1 mutations are sufficient to initiate TMD, possibly due to disinhibition of the E2F pathway. How genes in the Down syndrome critical region (DSCR) of chromosome 21 affect DS hematopoiesis remains incompletely understood. One such gene, DYRK1A (dual-specificity tyrosine-regulated kinase 1A), has been reported to negatively regulate cell cycle progression by phosphorylation and subsequent destabilization of type D cyclins. In contrast, prior T21 murine studies demonstrate DYRK1A cooperates with GATA1s to promote megakaryopoiesis. We studied DYRK1A in a human T21 induced pluripotent stem cell (iPSC) model and demonstrate that DYRK1A has an anti-proliferative effect in the context of GATA1s.
Results: We generated isogenic T21 WT GATA1 or GATA1 N-terminus truncated (GATA1s) iPSCs by reprogramming peripheral blood mononuclear cells from TMD patients. We used CRISPR/Cas9 gene editing to generate DYRK1A two allele knockdown or three allele knockout (KO) iPSC lines, with or without the GATA1s mutation. Hematopoietic differentiation was performed by embryoid body (EB) formation followed by colony formation assay or liquid culture.
In both T21/WT GATA1 and T21/GATA1s backgrounds, DYRK1A disruption led to decreased absolute progenitor production. Progenitors with DYRK1A KO had comparable hematopoietic colony formation compared to WT DYRK1A. However, in the context of GATA1s, DYRK1A KO progenitors had enhanced megakaryocyte and myeloid proliferation by 2- and 1.5-fold in liquid culture assays, respectively. EDU and Ki67 cell proliferation assays showed that DYRK1A KO in T21/GATA1s megakaryocytes had significantly increased cell proliferation but did not have the same effect on T21/WT GATA1 cells. We also observed that T21/GATA1s/DYRK1A KO was associated with aberrant megakaryocyte maturation and activation as measured by CD41 MFI and PAC-1 binding, respectively.
To understand the mechanisms driving enhanced proliferation in T21/GATA1s/DYRK1A KO megakaryocytes, RNA-sequencing and gene set enrichment analysis (GSEA) were performed on FACS-purified megakaryocytes. GSEA showed enrichment for genes related to cell proliferation and cell division, as well as enriched E2F targets, DREAM targets and G2/M checkpoint genes in T21/GATA1s/DYRK1A KO megakaryocytes. Differential gene expression demonstrated that Cyclin D2 was the top-ranked upregulated gene, indicating enhanced cell cycle G1/S transition and E2F signaling activation. Hematopoietic gene GATA2 was also significantly increased in T21/GATA1s/DYRK1A KO megakaryocytes.
Western blot analysis showed that compared to T21/WT GATA1, T21/GATA1s megakaryocytes showed relatively higher expression levels of pro-proliferative genes. All 3 type D cyclins (1, 2, 3) showed increased expression in the presence of DYRK1A KO, as well as subsequent hyperphosphorylation of RB that allows release of E2F to activate its downstream targets. DYRK1A KO suppressed cyclin D2 (T280) and D3 (T283) phosphorylation, indicating increased protein stability. Other pro-proliferative genes, such as cyclin A2, cyclin B1, and GATA2 were increased in DYRK1A KO, especially in the context of GATA1s. These data are consistent with the hematopoietic phenotypes described above and suggest that DYRK1A knockout and GATA1s have cooperative functions.
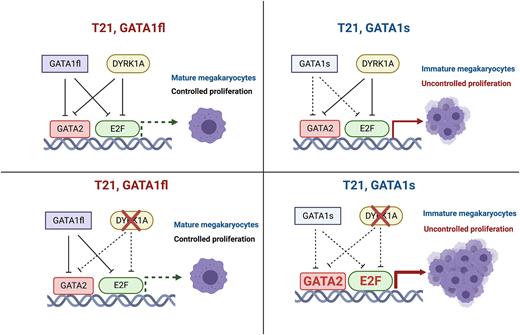
Conclusion: Our studies reveal distinct roles of DYRK1A in human DS hematopoietic progenitors and committed megakaryocytes in the context of T21 and truncated GATA1s. Our findings suggest that DYRK1A is necessary for normal hematopoietic progenitor production regardless of GATA1 status but specifically has a role in inhibiting megakaryocyte proliferation in the context of GATA1s. DYRK1A may cooperate with WT GATA1 to control megakaryopoiesis in T21 by inhibiting both GATA2 and E2F pathways. Loss of DYRK1A, combined with GATA1s leads to aberrant megakaryopoiesis, resulting in proliferative, immature megakaryocytes (Figure). In contrast to prior murine studies, our findings suggest DYRK1A inhibition would not be a potential therapeutic option for DS-AMKL.
Disclosures
French:Platelet Biogenesis: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal